Bereit, Ihr schönstes Ich zu entdecken? Finden Sie eine Cynosure Praxis in Ihrer Nähe.
Finden Sie eine PraxisDas SculpSure System Behandlungen zur nicht-invasiven Körperkonturierung reduziert hartnäckige Fettpölsterchen ohne Operation und ohne Ausfallzeit.

Mit unserer bewährten SculpSure Technologie können unerwünschte Fettzellen in nur 25 Minuten pro Behandlung reduziert werden. Das SculpSure System zur nicht-invasiven Körperkonturierung reduziert hartnäckige Fettpölsterchen ohne Operation und ohne Ausfallzeit.
SculpSure ist das weltweit erste von der FDA zugelassene Lasergerät für die nicht-invasive Lipolyse an Bauch, Hüften, Rücken, inneren und äußeren Oberschenkeln sowie unter dem Kinn (submental).
Unser Submental-Applikator eignet sich perfekt zur präzisen und effektiven Doppelkinnbehandlung für ein schlankeres Erscheinungsbild von Kinn und Hals. SculpSure Submental macht SculpSure zu einem Komplettsystem für die nicht-invasive Körperkonturierung.
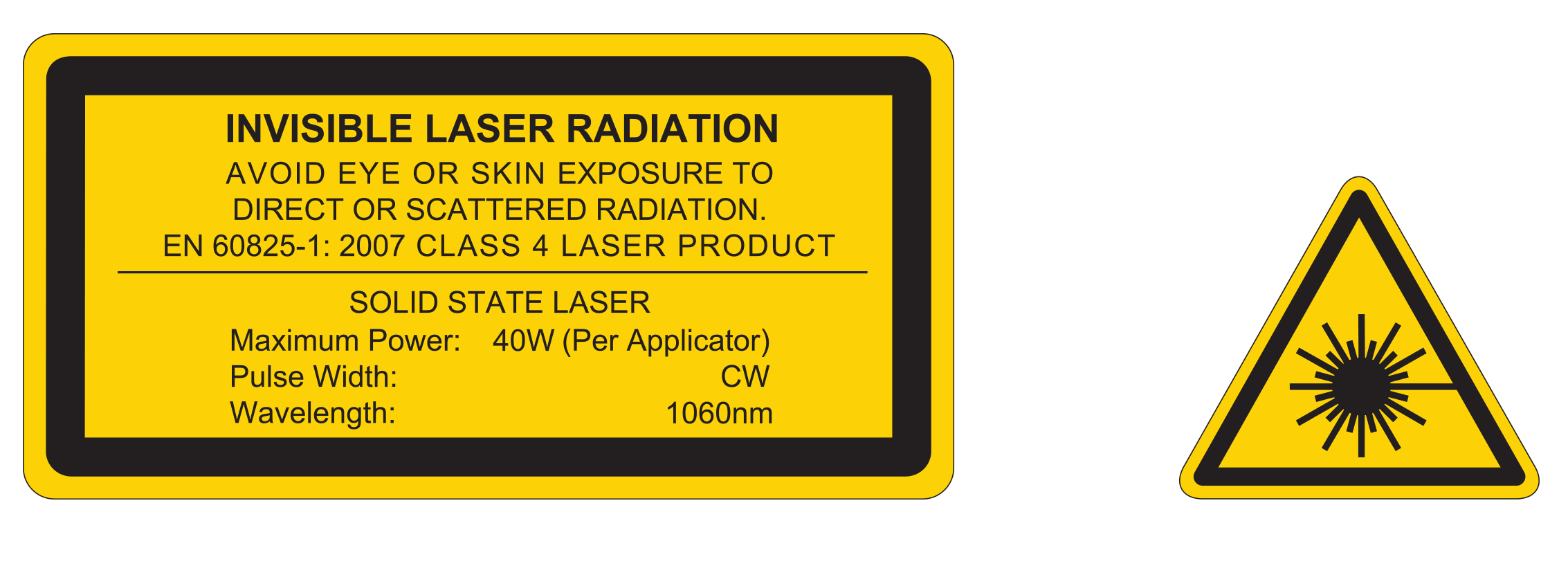
**When using the petite mask for non-invasive lipolysis of the submental area
Durch die Affinität der 1060 nm Wellenlänge für Fettgewebe und eine zugleich minimale Absorption in der Dermis ermöglicht SculpSure eine wirksame Behandlung von Problemzonen in nur 25 Minuten pro Behandlung. Im Laufe der Zeit werden die zerstörten Fettzellen auf natürliche Weise aus dem Körper ausgeschieden. Die ersten Ergebnisse sind bereits nach 6 Wochen sichtbar, der optimale Behandlungserfolg ist in nur 12 Wochen zu sehen.
1Klinische Archivdaten.
SculpSure für den Körper (Hüften, Bauch, Oberschenkel und Rücken) wurde für Kunden mit einem BMI von maximal 30 entwickelt. Die Konturierung eines Doppelkinns ist bis zu einem BMI von 49 möglich. Es müssen jedoch auch die Körperzusammensetzung eines jeden Patienten und seine Erwartungen berücksichtigt werden.
Jeder Patient ist zwar anders, aber Studien haben gezeigt, dass bis zu 24 % der Fettzellen im behandelten Bereich zerstört werden können.*
*Average reduction in fat volume following single treatment as measured by MRI; Clinical and Histological Evaluations of a 1060nm Laser Device for Non-Invasive Fat Reduction, John W. Decorato, M.D., FACS. Rafael Sierra, Ph.D., Bo Chen, Ph.D., Westford, MA, 2014.
Die behandelten Fettzellen werden dauerhaft zerstört und regenerieren sich nicht mehr. SculpSure wurde für Patienten mit gesundem Lebensstil entwickelt, die jedoch mit hartnäckigen Fettpolstern in behandelbaren Bereichen wie Hüften, Bauch, inneren und äußeren Oberschenkeln, Rücken und unter dem Kinn zu kämpfen haben. Solange es zu keiner signifikanten Gewichtszunahme kommt, halten die SculpSure Ergebnisse an.
Bei vielen Patienten sind erste Ergebnisse bereits sechs Wochen nach der Behandlung sichtbar, da der Körper beginnt, die zerstörten Fettzellen über das Lymphsystem abzutransportieren. Optimale Ergebnisse sind in der Regel 12 Wochen nach der letzten Behandlung zu sehen.
Bei den meisten Patienten sind mehrere Behandlungen bis zum gewünschten Ergebnis erforderlich. Sechs Wochen nach der Behandlung sind Ergebnisse sichtbar, optimale Ergebnisse normalerweise nach 12 Wochen.
SculpSure wird durch umfangreiche klinische Daten gestützt.
Decorato JW, Sierra R, Chen, B. Clinical and Histological Evaluations of a 1060nm Laser Device for Non-Invasive Fat Reduction. Paper präsentiert am: 2014 Annual American Society for Laser Medicine and Surgery Conference; April 2-6; Phoenix, AZ.
Katz B, Doherty S. A multicenter study of the safety and efficacy of a non-invasive 1060 nm diode laser for fat reduction of the flanks. Paper präsentiert am: Jahreskongress 2015 der American Society for Laser Medicine and Surgery; 22.-26. April; Kissimmee, FL.
Bass L, Doherty S. Non-Invasive Fat Reduction of the Abdomen with a 1060nm Diode Laser. Paper präsentiert am: Jahreskongress 2015 der American Society for Laser Medicine and Surgery; 22.-26. April; Kissimmee, FL.
Decorato, J. W., Chen, B., & Sierra, R. (2017). Subcutaneous adipose tissue response to a non-invasive hyperthermic treatment using a 1060 nm laser. Lasers in Surgery and Medicine, 49(5), 480-489. doi:10.1002/lsm.22625.
https://www.ncbi.nlm.nih.gov/pubmed/28103642
Schilling, L., MD, Saedi, N., MD, & Weiss, R., MD. (2017). 1060 nm Diode Hyperthermic Laser Lipolysis: The Latest in Non-Invasive Body Contouring. Journal of Drugs in Dermatology, 16(1), 48-72.
http://jddonline.com/articles/dermatology/S1545961616P0048X
Das SculpSure Gerät ist ein nicht-invasives Laser-Körperkonturierungssystem, das darauf abzielt, Fettzellen durch eine nicht-invasive Lipolyse an Bauch, Hüften (Flanken), Rücken, inneren und äußeren Oberschenkeln sowie unter dem Kinn (submental) dauerhaft zu eliminieren. Die Ergebnisse können im Einzelfall variieren und sind nicht garantiert. SculpSure® Behandlungen sind nicht zur Gewichtsabnahme oder für fettleibige Menschen vorgesehen. Es können geringfügige Nebenwirkungen wie vorübergehende Empfindlichkeit, Schwellungen oder Gewebeverhärtungen im Behandlungsbereich auftreten. Bitte fragen Sie Ihren Arzt, ob eine Behandlung mit SculpSure für Sie geeignet ist.

Dr. med. David McDaniel, Virginia Beach, VA“SculpSure bietet eine einzigartige nicht-invasive Methode für die Zerstörung von Fettzellen mit Laserenergie, die dann auf natürliche Weise über das Lymphsystem des Körpers ausgeschieden werden. Wir haben mit dieser Behandlung gute Ergebnisse am Körper wie auch unter dem Kinn erzielt.”
Ihr Erfolg ist unsere Priorität. Deshalb bieten wir Online-Support rund um die Uhr, komplette Marketing-Kits, Schulung und Weiterentwicklung sowie vieles mehr, damit Sie Ihre langfristigen Ziele erreichen.

Das SculpSure System Behandlungen zur nicht-invasiven Körperkonturierung reduziert hartnäckige Fettpölsterchen ohne Operation und ohne Ausfallzeit.
Cynosure ist hier, um Ihre Praxis in jeder Hinsicht unterstützen. Wir sind an strategisch günstigen Standorten in Asien und Europa präsent und unterhalten Beziehungen zu Vertriebspartnern auf fünf Kontinenten. In den USA stehen unsere Kundendienstmitarbeiter Ärzten rund um die Uhr zur Verfügung.